Abstract
Immunotherapies have transformed the clinical care of patients with cancer. Bispecific T cell engagers (TCEs) have recently entered early-phase clinical trials of multiple myeloma (MM) and shown remarkable response rates even in heavily pretreated patients. However, T cells are heterogeneous with respect to phenotype, function and specificity for tumor antigens and currently we have limited understanding how to identify and monitor tumor specific T cells in hematological malignancies. It is furthermore unclear why individual patients fail to elicit an antitumor immune response upon treatment with TCEs and whether a persistent T cell response to TCEs relies on reinvigoration of pre-existing tumor-infiltrating lymphocytes or on recruitment of novel T cells.
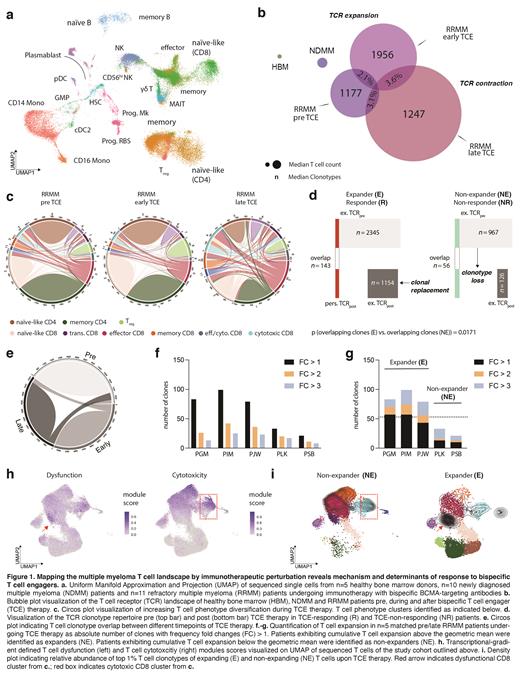
Here we performed longitudinal paired single-cell RNA and T cell receptor (TCR) sequencing on >100,000 immune cells from patients with MM before, during and after TCE therapy. We defined transcriptional gradients of MM-infiltrating immune cells between n=5 healthy bone marrow donors, n=10 newly diagnosed MM patients and n=11 refractory MM patients undergoing immunotherapy with bispecific BCMA-targeting antibodies. By tracking T cell clones over time using their TCR as individual barcode, we further integrated these longitudinal in vivo data with protein-level analysis and functional validation in MM bone-marrow cultures exposed to TCEs.
Refractory MM patients exhibited a highly individual bone-marrow immune composition, that was significantly perturbed compared to healthy or diseased, but therapy-naïve bone marrow. We observed that the inter-patient heterogeneity in the T cell landscape composition is superimposed by conserved TCR repertoire dynamics forming a trajectory between early anti-tumor effector states and exhaustion. In all patients, we observed a dichotomy of TCE-responsive versus TCE-refractory T cell clones. Longitudinal tracking of TCE-responsive T cell clones and their transcriptional phenotypes revealed coupling of tumor recognition, clonal expansion and T cell dysfunction marked by expression of cytotoxicity (GZMB, GNLY) and terminal exhaustion markers, such as TOX and CD39. Significant clonal replacement of T cells was evident in n=5 clinically responding patients with MM throughout continued TCE therapy and driven by a subset of non-exhausted, naïve-like CD8 + T cells. The top 1% TCE-responsive clones were fate-determined and either followed a memory-exhaustion or cytotoxicity trajectory. Patients who did not respond to TCE therapy exhibited a dysfunctional T cell landscape before therapy that limited clonal expansion and TCR persistence. As proof-of-concept, we matched single-cell profiling data of n=10 individual patients with protein-level analysis and functional validation of TCE-driven T cell expansion in vitro, providing the first signals of preferential expansion of specific fate- and avidity-determined clones upon TCE-mediated stimulation.
We propose the mode of action of TCE therapy in MM to be driven by pre-existing T cell fate commitments that determine clonotype diversification and persistence, and ultimately, clinical response. Our results further demonstrate that clinical TCE response derives from a distinct repertoire of pre-existing T cell clones, whereas other clonotypes are functionally excluded from the repertoire and subsequently lost during therapy. We define the determinants of response to TCE treatment to be inherent to the individual's T cell repertoire before therapy. Our results provide the rationale for response prediction and monitoring of future immunotherapy approaches in MM patients beyond TCE therapy.
Neri: BMS: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; Amgen: Consultancy, Honoraria. Goldschmidt: Amgen: Consultancy, Honoraria, Other: Grants and/or Provision of Investigational Medicinal Product, Research Funding; Adaptive Biotechnology: Consultancy; Celgene: Consultancy, Honoraria, Other: Grants and/or Provision of Investigational Medicinal Product, Research Funding; BMS: Consultancy, Honoraria, Other: Grants and/or Provision of Investigational Medicinal Product, Research Funding; Chugai: Honoraria, Other: Grants and/or Provision of Investigational Medicinal Product, Research Funding; GSK: Honoraria; Incyte: Research Funding; Janssen: Consultancy, Honoraria, Other: Grants and/or Provision of Investigational Medicinal Product, Research Funding; Johns Hopkins University: Other: Grant; Molecular Partners: Research Funding; MSD: Research Funding; Mundipharma: Research Funding; Novartis: Honoraria, Research Funding; Dietmar-Hopp-Foundation: Other: Grant; Sanofi: Consultancy, Honoraria, Other: Grants and/or Provision of Investigational Medicinal Product, Research Funding; Takeda: Consultancy, Research Funding. Weinhold: Sanofi: Honoraria. Raab: Abbvie: Consultancy, Honoraria; Roche: Consultancy; GSK: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees. Bahlis: Amgen: Consultancy, Honoraria; Karyopharm: Consultancy, Honoraria; Genentech: Consultancy; Janssen: Consultancy, Honoraria; BMS/Celgene: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; GlaxoSmithKline: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal